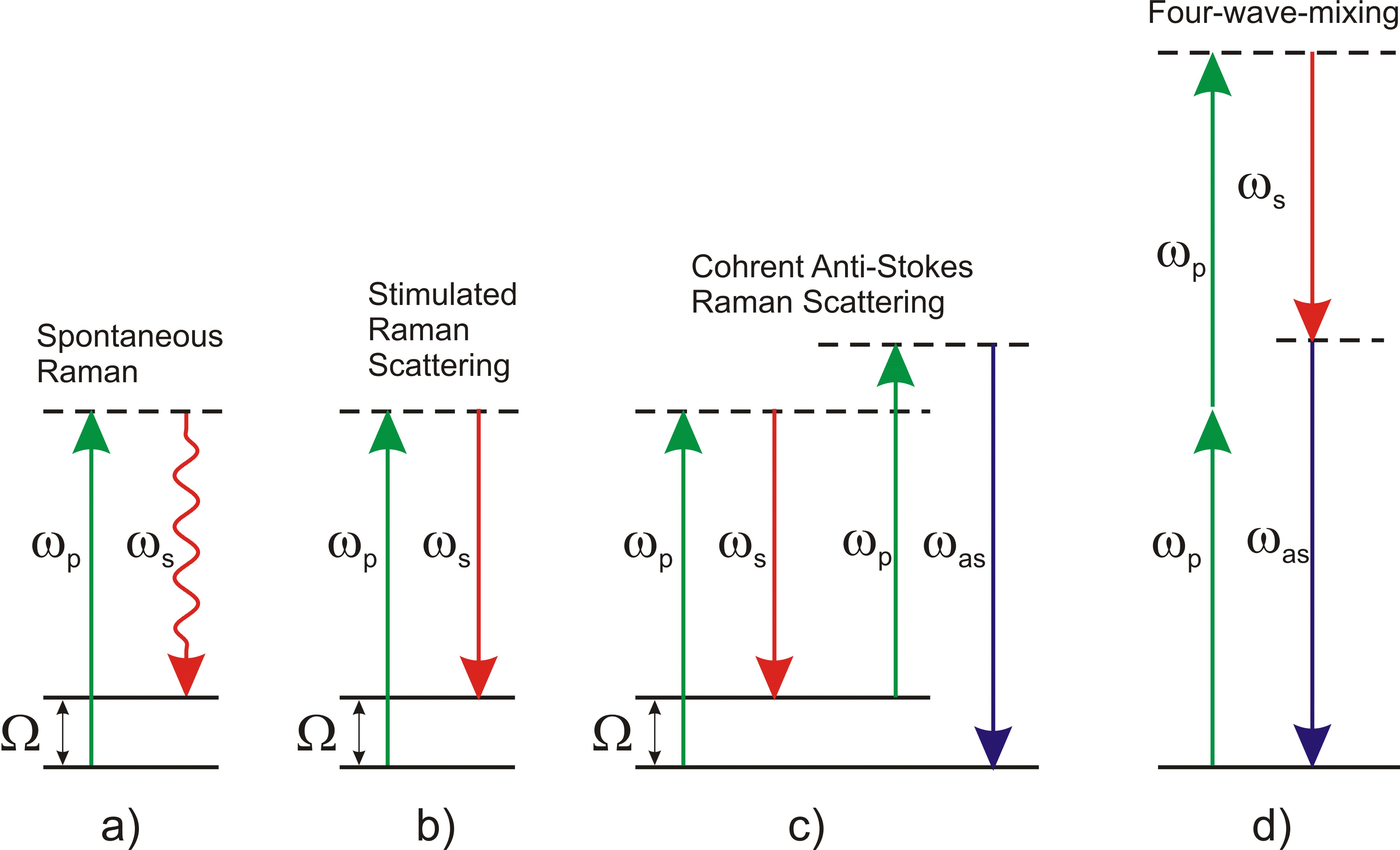
Optical microscopy has the unique capability of imaging biological samples with resolution down to the few hundred nanometers range. Despite its very high sensitivity, down to the single molecule limit, fluorescence microscopy requires sample staining with fluorescent marker molecules. Such labels often have sizes comparable to the molecules under study, thus altering the biological functionality. Raman scattering microscopy overcomes this limitation by providing an intrinsic contrast mechanism, based on the characteristic vibrational frequencies of the investigated molecule, allowing for noninvasive, labelfree imaging of tissues and cells. While spontaneous Raman scattering (Fig. 1a) yields weak signals and thus limited imaging speeds, the sensitivity can be boosted by many orders of magnitude by the use of coherent Raman scattering (CRS).
In Stimulated Raman Scattering (SRS, see Fig. 1b), pump and Stokes pulses are focused on the sample. Amplification of the Stokes pulse (and simultaneous depletion of the pump) occurs via stimulated emission from a virtual state to the probed vibrational state whenever the pump-Stokes frequency difference matches a vibrational mode Ω, thus providing a chemically specific signature that can be used to uniquely identify the molecule. Experimentally, either stimulated-Raman gain (SRG) of the Stokes or stimulated-Raman loss (SRL) of the pump can be measured. The main advantage of SRS is the suppression of the nonresonant background (see below), which greatly simplifies the interpretation of the images. On the other hand, the SRG/SRL signals are quite low (typically below 10−4), requiring the implementation of highly sensitive transient absorption setups.
In Coherent Anti-Stokes Raman Scattering (CARS, see Fig. 1c) the input pulses are the same as in SRS but the anti-Stokes signal at frequency at ωas=2ωp−ωs is detected. Respect to SRS, it is a background-free technique (no complex demodulation schemes are required) but it suffers from the presence of a strong nonresonant background due to the four-wave mixing process between the pump and Stokes pulses (see Fig. 1d). Such a background does not carry chemically specific information and, at low concentrations of the target molecules, can distort and even overwhelm the resonant signal.
We developped a novel CARS/SRS set-up in which multiple continuously tunable narrowband synchronized phase-coherent pulses are synthesized by spectral compression of femtosecond pulses emitted by a single highly compact fiber laser (see Fig. 2). Spectral compression of the broadband femtosecond pulses is achieved by generating narrowband second harmonic (SH) pulses in crystals with large group-delay mismatch between the fundamental frequency and the SH pulses. Such a technique was recently demonstrated to lead to conversion efficiencies in excess of 30% together with compression factors as high as 100.This approach allows for a dramatic simplification, downsizing, and cost reduction with respect to current implementations of SRS. In addition, it offers a much higher flexibility when implementing twocolor or multicolor imaging.
Read more:
- Gambetta, A., Kumar, V., Grancini, G., Polli, D., Ramponi, R., Cerullo, G., Marangoni, M. “Fiber-format stimulated-Raman-scattering microscopy from a single laser oscillator” (2010) Optics Letters, 35 (2), pp. 226-228.
- M. Marangoni, A. Gambetta, C. Manzoni, V. Kumar, R. Ramponi, and G. Cerullo, “Fiber-format CARS spectroscopy by spectral compression of femtosecond pulses from a single laser oscillator” Opt. Lett. 34, 3262 (2009).